Custom RCV Kit Development
-
Custom RCV Kit Development
For quality control and long-term monitoring of gene therapy products using viral vectors, detection of replication-competent viruses (RCV) is essential.
CellSafe develops custom detection and quantification kits tailored to the specific viral vector sequences of each client. -
Service Items
- Custom primer and probe design based on the client’s vector sequence
- Comprehensive Validation Package: LOD, LOQ, Recovery, linearity, specificity, and matrix effect verification
- Regulatory Submission Package: Validation protocol & report compliant with ICH Q2 guidelines
- Multiplex Options: Cross-contamination check using the positive control
- Sample Pretreatment & DNA Extraction Kits: Optimized pretreatment procedures tailored to each sample type
-
Performance Improvement
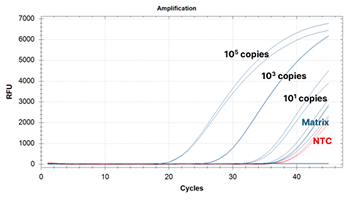
Custom RCV Kit Performance Improvement Example: Before Improvement Image 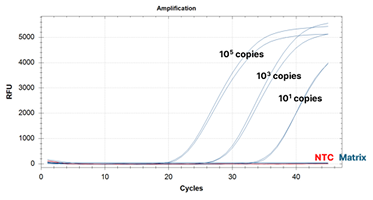
ustom RCV Kit Performance Improvement Example: After Improvement Image -
Service Workflow
-
Commercialized Kit
Testing -
NDA
/Contract Signing -
Primer Design
-
Performance
Test -
Prototype
Testing -
Reference
Material Production -
Validation
& Documentation -
Application
Complete
-
-
Turnaround Time
- Average: 4 months (varies by service items)
