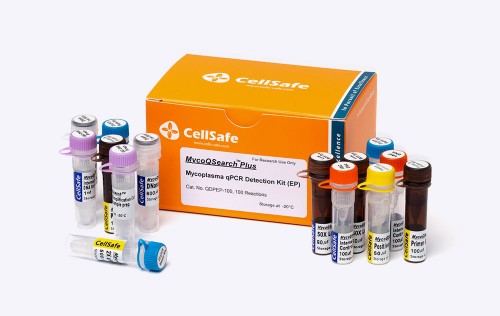
Product.
Detection
MycoQSearch™ Mycoplasma qPCR Detection Kit
This kit is designed to detect mycoplasma contamination in production cell lines and biopharmaceuticals through real-time PCR with high sensitivity. This product can be used as an alternative to conventional culture methods especially for the qualification of ATMPs.
QD-100, QDEP-100
Ordering Information
Product Overview
Features
- Mycoplasma test in production cell-lines and biopharmaceuticals(Compliance with Korea MFDS, EP and JP)
- Real-time quantitative PCR(qPCR) using Hydrolysis probe
(FAM labeled probe: mycoplasma, HEX labeled probe: IAC) - High Sensitivity : 1~10 copies/reaction
- Specificity : Do not detect other bacterial species with close phylogenetic relation to mycoplsma
- UDG system : Prevention of carry-over contamination
Results
Amplification Result
Tested with Quantitative M. orale Genomic DNA (Cat. No. QOGD, CellSafe)
Document
Certificates of analysis
Material Safety Data Sheet
Product FAQ
-
Q
What is the difference between MycoQSearch and MycoQSearch Plus?A
The Plus kit includes the functionality of detecting cross-contamination caused by positive control DNA using the Cy5 channel. This feature helps identify contamination that may occur due to positive control DNA during the experiment.
-
Q
Which channels should be selected on a real-time PCR instrument?A
This product uses the hydrolysis probe method and requires all two channels per well: FAM and HEX. If the instrument does not have a HEX channel, VIC may be used as a substitute.
-
Q
Is MycoQSearch compliant with regulatory standards (e.g., EP, JP, MFDS) for mycoplasma testing?A
Yes. MycoQSearch is suitable for mycoplasma testing for quality control of biologics, such as Cell and gene therapy. It meets required sensitivity with detection limits of 10CFU/mL.
For Technical support for method validation, please contact us via CellSafe website.